Common sensory domains in transmembrane receptors for diverse signal transduction pathways in Bacteria and Archaea
Abstract
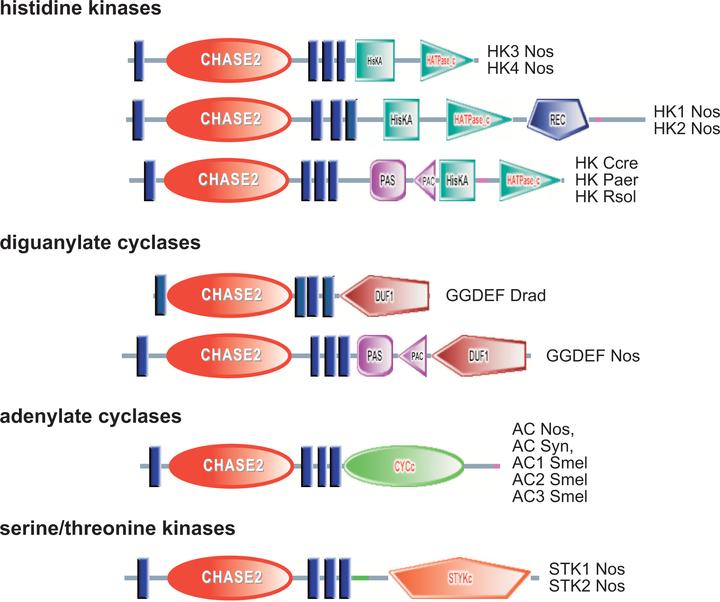
Transmembrane receptors in microorganisms, such as sensory histidine kinases and methyl-accepting chemotaxis proteins, are molecular devices for monitoring environmental changes. We report here that sensory domain sharing is widespread among different classes of transmembrane receptors. We have identified two novel conserved extracellular sensory domains, named CHASE2 and CHASE3, that are found in at least four classes of transmembrane receptors: histidine kinases, adenylate cyclases, predicted diguanylate cyclases, and either serine/threonine protein kinases (CHASE2) or methyl-accepting chemotaxis proteins (CHASE3). Three other extracellular sensory domains were shared by at least two different classes of transmembrane receptors: histidine kinases and either diguanylate cyclases, adenylate cyclases, or phosphodiesterases. These observations suggest that microorganisms use similar conserved domains to sense similar environmental signals and transmit this information via different signal transduction pathways to different regulatory circuits: transcriptional regulation (histidine kinases), chemotaxis (methyl-accepting proteins), catabolite repression (adenylate cyclases), and modulation of enzyme activity (diguanylate cyclases and phosphodiesterases). The variety of signaling pathways using the CHASE-type domains indicates that these domains sense some critically important extracellular signals.